AI-created image
Statements (25)
| Predicate | Object |
|---|---|
| gptkbp:instanceOf |
gptkb:Atom
|
| gptkbp:appliesTo |
gptkb:hydrogen_atom
|
| gptkbp:category |
atomic physics
history of science |
| gptkbp:describes |
structure of atom
|
| gptkbp:explains |
hydrogen spectral lines
|
| gptkbp:influencedBy |
gptkb:Rutherford_model
|
| gptkbp:limitation |
cannot explain spectra of multi-electron atoms
does not explain Stark effect does not explain Zeeman effect does not account for electron wave nature |
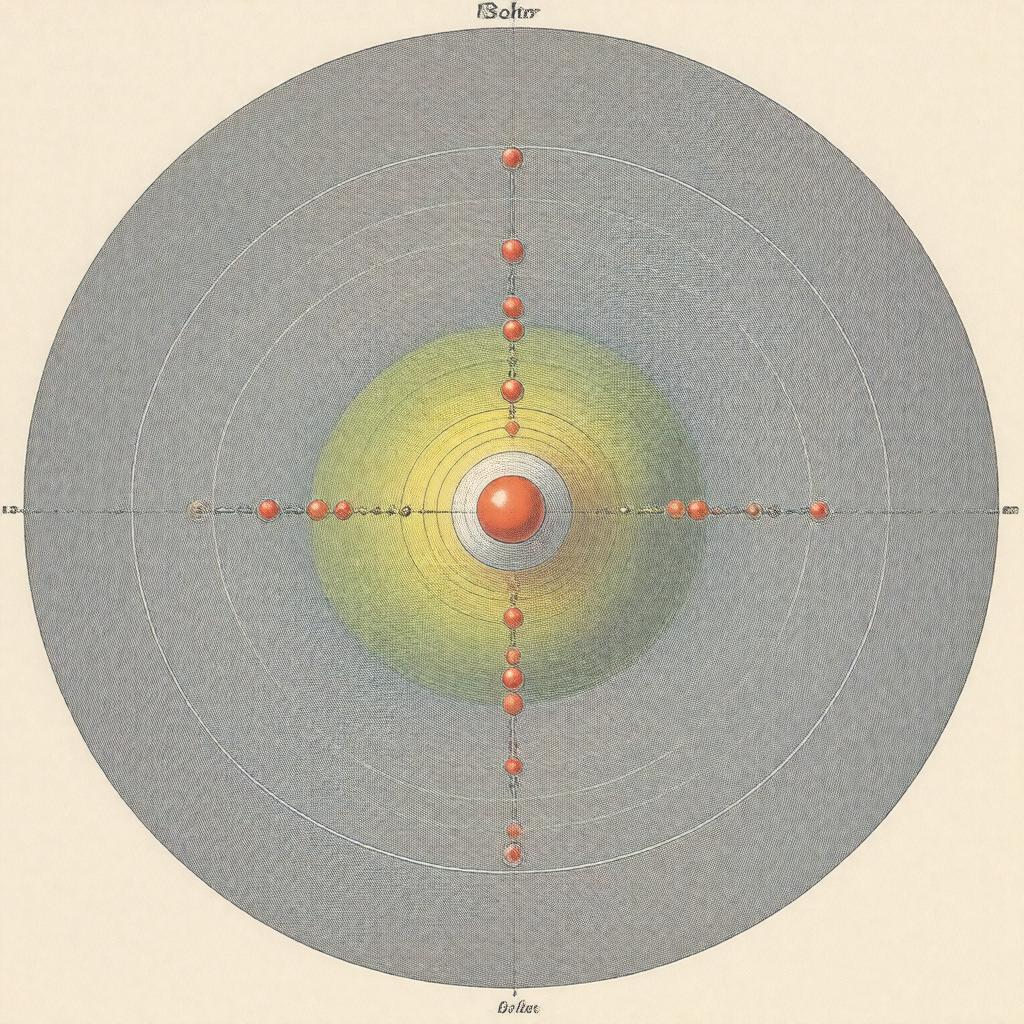
| gptkbp:postulates |
electrons orbit nucleus in fixed paths
energy levels are quantized electrons emit or absorb energy when changing orbits |
| gptkbp:proposedBy |
gptkb:Niels_Bohr
|
| gptkbp:relatedTo |
gptkb:Schrödinger_equation
gptkb:quantum_field_theory gptkb:Rutherford_atomic_model gptkb:Planck's_constant energy quantization |
| gptkbp:replacedBy |
gptkb:quantum_mechanical_model
|
| gptkbp:yearProposed |
1913
|
| gptkbp:bfsParent |
gptkb:Bohr_model
|
| gptkbp:bfsLayer |
4
|
| http://www.w3.org/2000/01/rdf-schema#label |
Bohr atomic model
|